How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review
- Research commodity
- Open Admission
- Published:
How long do nosocomial pathogens persist on inanimate surfaces? A systematic review
BMC Infectious Diseases volume 6, Article number:130 (2006) Cite this article
Abstract
Background
Inanimate surfaces take frequently been described as the source for outbreaks of nosocomial infections. The aim of this review is to summarize information on the persistence of different nosocomial pathogens on inanimate surfaces.
Methods
The literature was systematically reviewed in MedLine without language restrictions. In addition, cited articles in a study were assessed and standard textbooks on the topic were reviewed. All reports with experimental testify on the elapsing of persistence of a nosocomial pathogen on any type of surface were included.
Results
Most gram-positive leaner, such as Enterococcus spp. (including VRE), Staphylococcus aureus (including MRSA), or Streptococcus pyogenes, survive for months on dry surfaces. Many gram-negative species, such every bit Acinetobacter spp., Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, Serratia marcescens, or Shigella spp., can too survive for months. A few others, such as Bordetella pertussis, Haemophilus influenzae, Proteus vulgaris, or Vibrio cholerae, withal, persist only for days. Mycobacteria, including Mycobacterium tuberculosis, and spore-forming leaner, including Clostridium difficile, can also survive for months on surfaces. Candida albicans as the most important nosocomial fungal pathogen tin survive up to 4 months on surfaces. Persistence of other yeasts, such as Torulopsis glabrata, was described to be similar (5 months) or shorter (Candida parapsilosis, xiv days). Virtually viruses from the respiratory tract, such as corona, coxsackie, influenza, SARS or rhino virus, can persist on surfaces for a few days. Viruses from the gastrointestinal tract, such as astrovirus, HAV, polio- or rota virus, persist for approximately ii months. Claret-borne viruses, such equally HBV or HIV, tin persist for more than one week. Herpes viruses, such equally CMV or HSV type 1 and two, have been shown to persist from just a few hours up to 7 days.
Conclusion
The nearly mutual nosocomial pathogens may well survive or persist on surfaces for months and can thereby be a continuous source of manual if no regular preventive surface disinfection is performed.
Groundwork
Within the global infection control community, there is an ongoing controversy most the appropriate handling of inanimate surfaces in hospitals in gild to forestall transmission of nosocomial pathogens inside an institution. Based on a lack of epidemiological data that would provide evidence of a benefit for the patient from surface disinfection (e.thousand., from a significant reduction of nosocomial infection rates), some scientists postulate that cleaning of surfaces with non-antimicrobial detergents is mostly sufficient [1]. Others prefer cleaning of surfaces with antimicrobial agents, based on data on the take chances of infection due to microbial contamination and potential transmission of nosocomial pathogens, at least in the immediate vicinity of patients [2–iv].
New guidelines on treatment of surfaces in hospitals accept into account more parameters which are considered to be relevant for preventing the transmission of nosocomial pathogens, such as the blazon of ward or the expected frequency of mitt contact with a surface [five, 6]. Irrespective of the divergent opinions regarding the appropriate treatment of surfaces, an important parameter for a fair scientific assessment remains, that is, the persistence of nosocomial pathogens on surfaces. The longer a nosocomial pathogen persists on a surface, the longer it may exist a source of transmission and thus endanger a susceptible patient or healthcare worker. The aim of this review was therefore to collect and assess the information that take been published in the last decades on persistence of all types of nosocomial pathogens on surfaces, both in the context of surface disinfection and the control of nosocomial outbreaks.
Methods
Search strategy
The literature was systematically reviewed in MedLine on the internet homepage of the National Library of Medicine without language restrictions. The search was washed on 29 December 2005 and covered all years available in MedLine. The following search terms were applied: persistence, survival, surface, fomite, bacteria, virus, pathogen, transmission, and nosocomial. In improver, the citations in each study institute during the master search were reviewed for potential relevance. Finally, standard textbooks on infection control, bacteriology and virology were examined for information.
Selecting studies
All reports with experimental evidence on the elapsing of persistence of a nosocomial pathogen on any type of inanimate surface were included. Information from textbooks was likewise included, even if the chapter itself did non contain experimental evidence. At to the lowest degree two of the investigators decided on the relevance of each report. Reports were not blinded to the investigators then that they knew the names of the authors of all studies.
Interpretation of studies
For a clinically relevant summary, all nosocomial pathogens were grouped according to their importance in causing infirmary-acquired hand-transmitted infections [7] and according to their manner of nosocomial transmission [eight]. The range of the reported elapsing of persistence was used as the principle upshot of the search for each nosocomial pathogen. In addition, parameters with potential influence on persistence were evaluated in all experimental studies.
Results
Persistence of bacteria
About gram-positive bacteria, such every bit Enterococcus spp. (including VRE), Staphylococcus aureus (including MRSA), or Streptococcus pyogenes survive for months on dry surfaces (Tabular array 1). In full general, at that place was no obvious difference in survival between multiresistant and susceptible strains of Staphylococcus aureus and Enterococcus spp. [9]. Only in one study was such a divergence suggested, but the susceptible strains revealed a very brief survival as such [10]. Many gram-negative species, such as Acinetobacter spp., Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, Serratia marcescens, or Shigella spp. can survive on inanimate surfaces even for months. These species are found among the most frequent isolates from patients with nosocomial infections [11]. A few others, such as Bordetella pertussis, Haemophilus influenzae, Proteus vulgaris, or Vibrio cholerae, still, persist merely for days (Table 1). Mycobacteria – including Mycobacterium tuberculosis and spore-forming bacteria, including Clostridium difficile – tin can also survive for many months on surfaces (Table 1).
Overall, gram-negative bacteria have been described to persist longer than gram-positive bacteria [12, 13]. Humid conditions improved persistence for almost types of bacteria, such as Chlamydia trachomatis [14], Listeria monocytogenes [15], Salmonella typhimurium [15], Pseudomonas aeruginosa [16], Escherichia coli [17], or other relevant pathogens [eighteen, nineteen]. Only Staphylococcus aureus was found to persist longer at low humidity [sixteen].
Low temperatures, e.g., 4°C or half dozen°C, also improved persistence of most types of leaner, such Listeria monocytogenes [15], Salmonella typhimurium [fifteen], MRSA [twenty], corynebacteria [21], Escherichia coli [17, 22], Helicobacter pylori [23], and Neisseria gonorrhoeae [24].
The type of examination material does not reveal a consistent event. Although some investigators report that the type of textile has no influence on the persistence [25, 26], other authors described a longer persistence on plastic [27, 28], and others still see a survival advantage on steel [29].
Other factors were rarely investigated and hence provide inconsistent results. Longer persistence has been described with higher inocula [28], in the presence of poly peptide [13], serum [13, 24], sputum [30], or without grit [ten].
Persistence of fungi
Candida albicans as the nigh important nosocomial fungal pathogen can survive up to 4 months on surfaces (Table two). Persistence of other yeasts was described to be like (Torulopsis glabrata v months) or shorter (Candida parapsilosis 14 days).
The presence of serum or albumin, a low temperature, and high humidity have been described as leading to longer persistence [31].
Persistence of viruses
Nearly viruses from the respiratory tract such equally corona-, coxsackie-, fluvirus, SARS, or rhinocerosvirus tin persist on surfaces for a few days. Viruses from the alimentary canal, such equally astrovirus, HAV, polio- and rotavirus persist for approximately 2 months. Claret-borne viruses, such equally HBV or HIV, can persist for more than one week. Herpes viruses such as CMV or HSV blazon 1 and 2 have been shown to persist from merely a few hours up to 7 days.
The influence of humidity on persistence has been described inconsistently. For entero- [32] and rhinovirus [33], high humidity was associated with longer persistence. HSV [34] and HAV [35] can persist longer at low humidity. For adeno- [32, 34], rota- [36, 37], and poliovirus [34, 35], alien results were reported.
For nearly viruses, such as astro- [38], adeno- [34], poliovirus [34], HSV [34], and HAV [35], depression temperature is associated with a longer persistence.
Inconsistent results are also reported for the influence of type of material. Some authors described that the type of material did not bear on the persistence of echo- [39], adeno- [39–41], parainfluenza- [39], rotavirus [41], RSV [39], polio- [41] or norovirus [42]. Other investigators institute that persistence was favored on non-porous surfaces for influenzavirus [43], on formica and gloves for RSV [44], and on a telephone receiver for FCV [45].
Other parameters for a longer persistence of viruses include the presence of fecal suspension [38] and a higher inoculum [46].
Persistence of other nosocomial pathogens
Cryptosporidium species have been reported to survive on dry out surfaces for only 2 hours [47].
Discussion
The nearly relevant nosocomial pathogens can persist on dry out inanimate surfaces for months. In addition to the duration of persistence, some studies have also identified factors influencing persistence. A low temperature, such as four°C or 6°C, was associated with longer persistence for nigh bacteria, fungi and viruses. High humidity (east.g., > 70%) was likewise associated with longer persistence for virtually leaner, fungi, and viruses, although for some viruses alien results were reported. A few studies also propose that a higher inoculum is associated with longer persistence. The blazon of surface material and the type of suspension medium, even so, reveal inconsistent data. Overall, a loftier inoculum of the nosocomial pathogen in a cold room with loftier relative humidity volition accept the best chance for long persistence.
In nigh reports with experimental show, persistence was studied on dry out surfaces using artificial contamination of a standardized blazon of surface in a laboratory. In near studies, bacteria were prepared in broth, h2o or saline. Viruses were usually prepared in a cell culture medium [48]. The main advantage is that the environmental conditions are consistent regarding temperature and air humidity. In addition, the effect of temperature or relative humidity can only be determined under controlled conditions, which are much easier to ensure in the laboratory. Even so, this may non always reflect the clinical situation, in which surfaces tin can exist simultaneously contaminated with various nosocomial pathogens and different types of body fluids, secretions etc. Still the question remains: what is the clinical evidence for the function of surfaces in nosocomial infections?
In hospitals, surfaces with paw contact are ofttimes contaminated with nosocomial pathogens [49–51], and may serve equally vectors for cross manual. A unmarried hand contact with a contaminated surface results in a variable degree of pathogen transfer. Transmission to hands was most successful with Escherichia coli, Salmonella spp., Staphylococcus aureus (all 100%) [52], Candida albicans (ninety%) [53], rhino virus (61%) [54], HAV (22% – 33%) [55], and rota virus (16%) [56, 57]. Contaminated hands can transfer viruses to v more surfaces [58] or 14 other subjects [59]. Contaminated hands can as well be the source of re-contaminating the surface, as shown with HAV [55, 58]. Compliance rates of healthcare workers in hand hygiene are known to be around 50% [vii]. Due to the overwhelming bear witness of low compliance with hand hygiene, the hazard from contaminated surfaces cannot be disregarded (Figure 1).
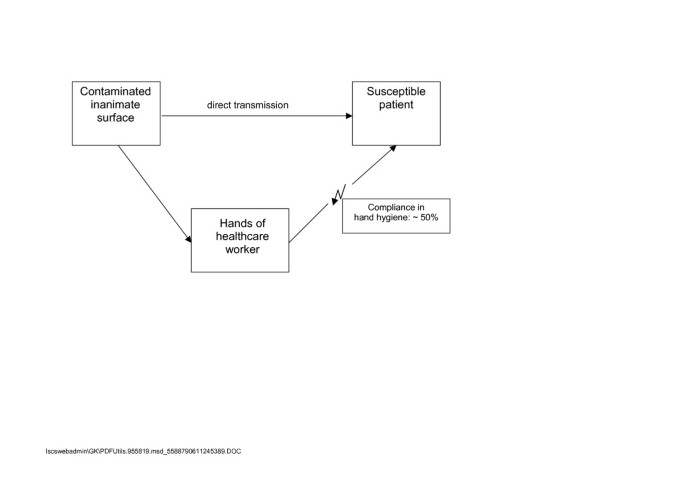
Common modes of transmission from inanimate surfaces to susceptible patients.
The main road of transmission is via the transiently contaminated hands of the healthcare worker [60–62]. An outbreak of nosocomial infections due to Acinetobacter baumannii in a neurosurgical intensive intendance unit may serve equally an example. A straight correlation was found betwixt the number of ecology isolates obtained during screening and the number of patients who were colonized or infected with the same strain during the same calender month [63].
During outbreaks, the environment may play a pregnant role for transmission of nosocomial pathogens, as suggested by observational testify. This has been described for various types of microorganisms, such as Acinetobacter baumannii [64–66], Clostridium difficile [67–69], MRSA [65, 70], Pseudomonas aeruginosa [4, 65], VRE [65, 71–77], SARS [78, 79], rota- [80, 81], and norovirus [82]. However, the testify to support a role of environmental contamination is not equally strong for all types of nosocomial pathogens. For Clostridium difficile, MRSA, and VRE, data are stronger than for other pathogens, such as Pseudomonas aeruginosa or Acinetobacter baumannii, of which multiple types were detected in the environs, and which did non always correlate with the caused strain [83].
The role of surface disinfection for the control of nosocomial pathogens has been a contentious issue for some fourth dimension [3]. Routine treatment of clean floors with diverse types of surface disinfectants (some of them had rather poor bactericidal activity) has been described to have no significant impact on the incidence of nosocomial infections [84]. Disinfection of surfaces in the immediate environment of patients, nevertheless, has been described to reduce acquisition of nosocomial pathogens such as VRE [85] or Acinetobacter baumannii [86]. It is therefore appropriate to control the spread of nosocomial pathogens at least in the straight inanimate environment of the patient past routine surface disinfection.
Conclusion
Near nosocomial pathogens can persist on inanimate surfaces for weeks or even months. Our review supports current guidelines which recommend a disinfection of surfaces in specific patient-care areas in lodge to reduce the risk of transmission of nosocomial pathogens from inanimate surfaces to susceptible patients.
References
-
Allerberger F, Ayliffe G, Bassetti M, Braveny I, Bucher A, Damani N, Daschner FD, Dettenkofer Thou, Ezpeleta C, Gastmeier P, Geffers C, Giamarellou H, Goldman D, Grzesiowski P, Gubina M, Haanen PE, Haydouchka I, Hubner J, Kalenic Southward, Van Knippenberg-Gordebeke Chiliad, Kranenburg AM, Krcmery 5, Kropec A, Kruger W, Lemmen South, Mayhall CG, Meester G, Mehtar S, Munzinger J, Muzlovic I, Ojajarvi J, Rüden H, Scott G, Shah P, Tambic-Andraszevic A, Unertl K, Voss A, Weist K: Routine surface disinfection in health care facilities: should nosotros do it?. American Periodical of Infection Control. 2002, thirty: 318-319. x.1067/mic.2002.127205.
-
Rutala WA, Weber DJ: Surface disinfection: should we do it?. Journal of Hospital Infection. 2001, 48: 64S-68S. 10.1016/S0195-6701(01)90017-9.
-
Cozad A, Jones RD: Disinfection and the prevention of infectious disease. American Journal of Infection Control. 2003, 31: 243-254. ten.1067/mic.2003.49.
-
Engelhart Due south, Krizek L, Glasmacher A, Fischnaller East, Marklein Yard, Exner Grand: Pseudomonas aeruginosa outbreak in a haematology-oncology unit of measurement associated with contaminated surface cleaning equipment. Periodical of Hospital Infection. 2002, 52: 93-98. x.1053/jhin.2002.1279.
-
Bearding: Anforderungen an dice Hygiene bei der Reinigung und Desinfektion von Flächen. Bundesgesundheitsblatt. 2004, 47: 51-61. 10.1007/s00103-003-0752-9.
-
Anonymous: Guidelines for environmental infection control in health-intendance facilities. MMWR - Morbidity & Mortality Weekly Written report. 2003, 52: i-44.
-
Kampf M, Kramer A: Epidemiologic background of hand hygiene and evaluation of the nigh important agents for scrubs and rubs. Clinical Microbiology Reviews. 2004, 17: 863-893. x.1128/CMR.17.iv.863-893.2004.
-
Aitken C, Jeffries DJ: Nosocomial spread of viral disease. Clinical Microbiology Reviews. 2001, 14: 528-546. 10.1128/CMR.14.3.528-546.2001.
-
Neely AN, Maley MP: Survival of enterococci and staphylococci on infirmary fabric and plastic. Periodical of Clinical Microbiology. 2000, 38: 724-726.
-
Wagenvoort JHT, Penders RJR: Long-term in-vitro survival of an epidemic MRSA phage-group III-29 strain. Journal of Hospital Infection. 1997, 35: 322-325. x.1016/S0195-6701(97)90229-2.
-
Rüden H, Gastmeier P, Daschner FD, Schumacher M: Nosocomial and community-acquired infections in Germany. Summary of the results of the first national prevalence written report (NIDEP). Infection. 1997, 25: 199-202. 10.1007/BF01713142.
-
Dickgiesser N: Untersuchungen über das Verhalten grampositiver und gramnegativer Bakterien in trockenem und feuchtem Milieu. Zentralblatt für Bakteriologie und Hygiene, I Abt Orig B. 1978, 167: 48-62.
-
Hirai Y: Survival of bacteria under dry conditions from a viewpoint of nosocomial infection. Journal of Hospital Infection. 1991, 19: 191-200. 10.1016/0195-6701(91)90223-U.
-
Novak KD, Kowalski RP, Karenchak LM, Gordon YJ: Chlamydia trachomatis tin can be transmitted past a nonporous plastic surface in vitro. Cornea. 1995, 14: 523-526.
-
Helke DM, Wong ACL: Survival and growth characteristics of Listeria monocytogenes and Salmonella typhimurium on stainless steel and Buna-Northward rubber. Periodical of Food Protection. 1994, 57: 963-968.
-
Gundermann KO: Untersuchungen zur Lebensdauer von Bakterienstämmen im Staub unter dem Einfluß unterschiedlicher Luftfeuchtigkeit. Zentralblatt für Bakteriologie und Hygiene, I Abt Orig B. 1972, 156: 422-429.
-
Williams AP, Avery LM, Killham M, Jones DL: Persistence of Escherichia coli O157 on farm surfaces under different environmental conditions. Journal of Applied Microbiology. 2005, 98: 1075-1083. x.1111/j.1365-2672.2004.02530.x.
-
Jawad A, Snelling AM, Heritage J, Hawkey PM: Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry out surfaces. Periodical of Clinical Microbiology. 1996, 34: 2881-2887.
-
Jawad A, Snelling AM, Heritage J, Hawkey PM: Exceptional desiccation tolerance of Acinetobacter radioresistens. Journal of Infirmary Infection. 1998, 39: 235-240. 10.1016/S0195-6701(98)90263-eight.
-
Noyce JO, Michels H, Keevil CW: Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare surround. Journal of Hospital Infection. 2006, 63: 289-297. 10.1016/j.jhin.2005.12.008.
-
Augustine JL, Renshaw HW: Survival of Corynebacterium pseudotuberculosis in axenic purulent exudate on common barnyard fomites. American Journal of Vetenary Inquiry. 1986, 47: 713-715.
-
Wilks SA, Michels H, Keevil CW: The survival of Escherichia coli O157 on a range of metal surfaces. International Journal of Food Microbiology. 2005, 105: 445-454. x.1016/j.ijfoodmicro.2005.04.021.
-
Boehmler Thou, Gerwert J, Scupin E, Sinell HJ: Zur Epidemiologie der Helicobacteriose des Menschen; Untersuchungen zur Überlebensfähigkeit des Erregers in Lebensmitteln. Deutsche Tierärztliche Wochenschrift. 1996, 103: 438-443.
-
Elmos T: Survival of Neisseria gonorrhoeae on surfaces. Acta Dermato-Venereologica. 1977, 57: 177-180.
-
Wendt C, Dietze B, Dietz E, Rüden H: Survival of Acinetobacter baumannii on dry out surfaces. Periodical of Clinical Microbiology. 1997, 35: 1394-1397.
-
Bale MJ, Bennett PM, Benninger JE, Hinton K: The survival of bacteria exposed to dessication on surfaces associated with farm buildings. Periodical of Applied Bacteriology. 1993, 75: 519-528.
-
Pérez JL, Gómez Eastward, Sauca M: Survival of gonococci from urethral belch on fomites. European Periodical of Clinical Microbiology and Infectious Diseases. 1990, ane: 54-55.
-
Neely AN: A survey of gram-negative bacteria survival on infirmary fabrics and plastics. Journal of Burn down Intendance and Rehabilitation. 2000, 21: 523-527.
-
Webster C, Towner KJ, Humphreys H: Survival of Acinetobacter on iii clinically related inanimate surfaces. Infection Command and Hospital Epidemiology. 2000, 21: 246-ten.1086/503214.
-
Smith CR: Survival of tubercle bacilli: the viability of dried tubercle bacilli in unfiltered roomlight, in the dark, and in the fridge. American Review of Tuberculosis. 1942, 5: 334-345.
-
Blaschke-Hellmessen R, Kreuz M, Sprung M: Umweltresistenz und natürliche Keimreservoire medizinisch bedeutsamer Sprosspilze. Zeitschrift für die gesamte Hygiene. 1985, 31: 712-715.
-
Hara J, Okomator S, Minekawa Y, Yamazaki K, Kase T: Survival and disinfection of adenovirus type nineteen and enterovirus seventy in ophthalmic practice. Japanese Journal of Ophthalmology. 1990, 34: 421-427.
-
Sattar SA, Karim YG, Springthorpe VS, Johnson-Lussenburg CM: Survival of human rhinovirus type 14 dried onto nonporous inanimate surfaces: effect of relative humidity and suspending medium. Canadian Journal of Microbiology. 1987, 33: 802-806.
-
Mahl MC, Sadler C: Virus survival on inanimate surfaces. Canadian Journal of Microbiology. 1975, 21: 819-823.
-
Mbithi JN, Springthorpe VS, Sattar SA: Effect of relative humidity and air temperature on survival of hepatitis A virus on ecology surfaces. Applied and Environmental Microbiology. 1991, 57: 1394-1399.
-
Ansari SA, Springthorpe VS, Sattar SA: Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Reviews of Infectious Diseases. 1991, 13: 448-461.
-
Sattar S, Lloyd-Evans N, Springthorpe VS: Institutional outbreaks of rotavirus diarrhoea: potential office of fomites and ecology surfaces as vehicles for virus transmission. Journal of Hygiene, Cambridge. 1986, 96: 277-289.
-
Abad FX, Villena C, Guix Due south, Caballero S, Pintó RM, Bosch A: Potential office of fomites in the vesicular transmission of homo astroviruses. Applied and Environmental Microbiology. 2001, 67: 3904-3907. 10.1128/AEM.67.9.3904-3907.2001.
-
Wladowetz VW, Dmitrijewa RA, Safjulin AA: Die Persistenz von Viren auf Oberflächen und die Anwendung der UV-Bestrahlung zur Virusdesinfektion. Zeitschrift für die gesamte Hygiene. 1974, vii: 173-176.
-
Gordon YJ, Gordon RY, Romanowski E, Araullo-Cruz TP: Prolonged recovery of desiccated adenoviral serotypes 5, 8, and xix from plastic and metal surfaces in vitro. Ophthalmology. 1993, 100: 1835-1839.
-
Abad FX, Pinto RM, Bosch A: Survival of enteric viruses on environmental fomites. Applied and Environmental Microbiology. 1994, threescore: 3704-3710.
-
d'Souza DH, Williams Thousand, Jean J, Sair A, Jaykus L: Persistence of Norwalk virus on environmental surfaces and its transfer to food: ; Washington, D.C.. 2003, American Society for Microbiology
-
Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH: Survival of influenza viruses an environmental surfaces. The Journal of Infectious Diseases. 1982, 146: 47-51.
-
Hall CB, Douglas RG, Geiman JM: Possible transmission by fomites of respiratory syncytial virus. The Journal of Infectious Diseases. 1980, 141: 98-102.
-
Clay Southward, Maherchandani S, Malik YS, Goyal SM: Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. American Journal of Infection Control. 2006, 34: 41-43. 10.1016/j.ajic.2005.05.013.
-
Faix RG: Comparative efficacy of handwashing agents against cytomegalievirus. Infection Command. 1987, viii: 158-162.
-
Weber DJ, Rutala WA: The emerging nosocomial pathogens Cryptosporidium, Escherichia coli O157:h7, Helicobacter pylori, and hepatitis C: epidemiology, environmental survival, efficacy of disinfection, and command measures. Infection Control and Hospital Epidemiology. 2001, 22: 306-315. 10.1086/501907.
-
Rzezutka A, Cook N: Survival of human being enteric viruses in the surroundings and food. FEMS Microbiology Reviews. 2004, 28: 441-453.
-
Bures South, Fishbain JT, Uyehara CF, Parker JM, Berg BW: Reckoner keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. American Journal of Infection Control. 2000, 28: 465-471. 10.1067/mic.2000.107267.
-
Catalano M, Quelle LS, Jeric PE, Di Martino A, Maimone SM: Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. Journal of Infirmary Infection. 1999, 42: 27-35. 10.1053/jhin.1998.0535.
-
Boyce JM, Potter-Bynoe G, Chenevert C, Rex T: Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infection Control and Hospital Epidemiology. 1997, 18: 622-627.
-
Scott East, Bloomfield SF: The survival and transfer of microbial contamination via cloths, easily and utensils. Journal of Applied Bacteriology. 1990, 68: 271-278.
-
Rangel-Frausto MS, Houston AK, Bale MJ, Fu C, Wenzel RP: An experimental model for study of Candida survival and manual in man volunteers. European Journal of Clinical Microbiology and Infectious Diseases. 1994, 13: 590-595. 10.1007/BF01971311.
-
Gwaltney JM, Hendley JO: Transmission of experimental rhinovirus infection by contaminated surfaces. American Journal of Epidemiology. 1982, 116: 828-833.
-
Mbithi JN, Springthorpe VS, Boulet JR, Sattar SA: Survival of hepatitis A virus on human being hands and its transfer on contact with breathing an inanimate surfaces. Journal of Clinical Microbiology. 1992, 30: 757-763.
-
Ward RL, Bernstein DI, Knowlton DR, Sherwood JR, Young EC, Cusack TM, Rubino JR: Prevention of surface-to-human transmission of rotaviruses by treatment with disinfectant spray. Periodical of Clinical Microbiology. 1991, 29: 1991-1996.
-
Ansari SA, Sattar SA, Springthorpe VS, Wells GA, Tostawaryk W: Rotavirus survival on homo hands and transfer of infectious virus to inanimate and nonporous inanimate surfaces. Journal of Clinical Microbiology. 1988, 26: 1513-1518.
-
Barker J, Vipond IB, Bloomfield SF: Furnishings of cleaning and disinfection in reducing the spread of norovirus contagion via environmental surfaces. Periodical of Hospital Infection. 2004, 58: 42-44. 10.1016/j.jhin.2004.04.021.
-
von Rheinbaben F, Schunemann Due south, Gross T, Wolff MH: Transmission of viruses via contact in a household setting: experiments using bacteriophage strain phiXI174 every bit a model virus. Journal of Hospital Infection. 2000, 46: 61-66. ten.1053/jhin.2000.0794.
-
Laborde DJ, Weigle KA, Weber DJ, Kotch JB: Upshot of fecal contamination on diarrheal illness rates in twenty-four hours-care centers. American Journal of Epidemiology. 1993, 138: 243-255.
-
Mermel LA, Josephson SL, Dempsey J, Parenteau S, Perry C, Magill North: Outbreak of Shigella sonnei in a clinical microbiology laboratory. Journal of Clinical Microbiology. 1997, 35: 3163-3165.
-
Farrington M, Ling J, Ling T, French GL: Outbreaks of infection with methicillin-resistant Staphylococcus aureus on neonatal and burns units of a new infirmary. Epidemiology and Infection. 1990, 105: 215-228.
-
Denton M, Wilcox MH, Parnell P, Green D, Keer V, Hawkey PM, Evans I, Potato P: Part of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit of measurement. Periodical of Hospital Infection. 2004, 56: 106-110. 10.1016/j.jhin.2003.10.017.
-
Fierobe L, Lucet JC, Decre D, Muller-Serieys C, Deleuze A, Joly-Guillou ML, Mantz J, Desmonts JM: An outbreak of imipenem-resistant Acinetobacter baumannii in critically ill surgical patients. Infection Control and Hospital Epidemiology. 2001, 22: 35-40. x.1086/501822.
-
Lemmen SW, Häfner H, Zolldann D, Stanzel S, Lütticken R: Distribution of multi-resistant Gram-negative versus Gram-positive bacteria in the hospital inanimate environment. Periodical of Hospital Infection. 2004, 56: 191-197. x.1016/j.jhin.2003.12.004.
-
Ling ML, Ang A, Wee M, Wang GC: A nosocomial outbreak of multiresistant Acinetobacter baumannii originating from an intensive care unit. Infection Command and Infirmary Epidemiology. 2001, 22: 48-49. 10.1086/501827.
-
Hanna H, Raad I, Gonzalez Five, Umphrey J, Tarrand J, Neumann J, Champlin R: Command of nosocomial Clostridium difficile transmission in bone marrow transplant patients. Infection Command and Hospital Epidemiology. 2000, 21: 226-228. ten.1086/501751.
-
Verity P, Wilcox MH, Fawley West, Parnell P: Prospective evaluation of ecology contamination by Clostridium difficile in isolation side rooms. Journal of Infirmary Infection. 2001, 49: 204-209. 10.1053/jhin.2001.1078.
-
Kaatz GW, Gitlin SD, Schaberg DR, Wilson KH, Kauffman CA, Seo SM, Fekety R: Acquisition of Clostridium difficile from the hospital environment. American Journal of Epidemiology. 1988, 127: 1289-1294.
-
Fitzpatrick F, Murphy OM, Brady A, Prout Due south, Fenelon LE: A purpose built MRSA cohort study. Journal of Infirmary Infection. 2000, 46: 271-279. x.1053/jhin.2000.0838.
-
Falk PS, Winnike J, Woodmansee C, Desai M, Mayhall CG: Outbreak of vancomycin-resistant enterococci in a burn unit. Infection Control and Hospital Epidemiology. 2000, 21: 575-582. 10.1086/501806.
-
Greyness JW, George RH: Experience of vancomycin-resistant enterococci in a children's hospital. Journal of Infirmary Infection. 2000, 45: 11-18. 10.1053/jhin.1999.0724.
-
McCarthy KM, Van Nierop Due west, Duse A, Von Gottberg A, Kassel Grand, Perovic O, Smego R: Control of an outbreak of vancomycin-resistant Enterococcus faecium in an oncology ward in South Africa: effective use of express resources. Journal of Infirmary Infection. 2000, 44: 294-300. 10.1053/jhin.1999.0696.
-
Hanna H, Umphrey J, Tarrand J, Mendoza Yard, Raad I: Management of an outbreak of vancomycin-resistant enterococci in the medical intensive care unit of measurement of a cancer center. Infection Control and Hospital Epidemiology. 2001, 22: 217-219. x.1086/501892.
-
Martinez JA, Ruthazer R, Hansjosten K, Barefoot Fifty, Snydman DR: Role of environmental contagion as a take a chance factor for conquering of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Athenaeum of Internal Medicine. 2003, 163: 1905-1912. 10.1001/archinte.163.16.1905.
-
Bonten MJM, Hayden MK, Nathan C, van Voorhis J, Matushek K, Slaughter Due south, Rice T, Weinstein RA: Epidemiology of colonization of patients and environment with vancomycin-resistant enterococci. The Lancet. 1996, 348: 1615-1619. 10.1016/S0140-6736(96)02331-viii.
-
Duckro AN, Blom DW, Lyle EA, Weinstein RA, Hayden MK: Transfer of vancomycin-resistant enterococci via health care worker hands. Archives of Internal Medicine. 2005, 165: 302-307. 10.1001/archinte.165.3.302.
-
Mukhopadhyay A, Tambyah PA, Singh KS, Lim TK, Lee KH: SARS in a hospital visitor and her intensivist. Journal of Infirmary Infection. 2004, 56: 249-250. 10.1016/j.jhin.2003.12.015.
-
Chen YC, Huang LM, Chan CC, Su CP, Chang SC, Chang YY, Chen ML, Hung CC, Chen WJ, Lin FY, Lee YT: SARS in hospital emergency room. Emerging Infectious Diseases. 2004, 10: 782-788.
-
Butz AM, Fosarelli P, Dick J, Cusack T, Yolken R: Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993, 92: 202-205.
-
Wilde J, Van R, Pickering L, Eiden J, Yolken R: Detection of rotaviruses in the day care environment by reverse transcriptase polymerase concatenation reaction. The Periodical of Infectious Diseases. 1992, 166: 507-511.
-
Chadwick PR, Beards G, Brown D, Caul EO, Cheesbrough J, Clarke I, Curry A, O'Brien S, Quigley Chiliad, Sellwood J, Westmoreland D: Direction of hospital outbreaks of gastro-enteritis due to small roundstructured viruses. Periodical of Infirmary Infection. 2000, 45: i-x. x.1053/jhin.2000.0662.
-
Hota B: Contamination, disinfection, and cantankerous-colonization: are hospital surfaces reservoirs for nosocomial infection?. Clinical Infectious Diseases. 2004, 39: 1182-1189. 10.1086/424667.
-
Dharan S, Mourouga P, Copin P, Bessmer G, Tschanz B, Pittet D: Routine disinfection of patients' environmental surfaces. Myth or reality?. Journal of Hospital Infection. 1999, 42: 113-117. 10.1053/jhin.1999.0567.
-
Hayden MK, Bonten MJM, Blom DW, Lyle EA, van de Vijver DAMC, Weinstein RA: Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clinical Infectious Diseases. 2006, 42: 1552-1560. 10.1086/503845.
-
Wilks 1000, Wilson A, Warwick S, Price Eastward, Kennedy D, Ely A, Millar MR: Control of an outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus colonization and infection in an intensive care unit of measurement (ICU) without closing the ICU or placing patients in isolation. Infection Control and Infirmary Epidemiology. 2006, 27: 654-658. ten.1086/507011.
-
Musa EK, Desai Due north, Casewell MW: The survival of Acinetobacter calcoaceticus inoculated on fingertips and on formica. Journal of Hospital Infection. 1990, fifteen: 219-227. 10.1016/0195-6701(90)90029-N.
-
Getchell-White SI, Donowitz LG, Groschel DH: The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infection Control and Hospital Epidemiology. 1989, 10: 402-407.
-
Hahn H, Arvand Thou: Bordetellen. Medizinische Mikrobiologie und Infektiologie. Edited by: Hahn H, Falke D, Kaufmann SHE and Ullmann U. 2001, Berlin, Springer, 320-325. 4th
-
Mitscherlich E, Marth EH: Microbial survival in the environment. 1984, Berlin, Springer
-
Boucher SN, Chamberlain AHL, Adams MR: Enhanced survival of Campylobacter jejuni in association with wood. Journal of Food Protection. 1998, 61: 26-xxx.
-
Kim KH, Fekety R, Batts DH, Brown D, Cudmore Grand, Silva J, Waters D: Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. The Periodical of Infectious Diseases. 1981, 143: 42-l.
-
Mulligan ME, George WL, Rolfe RD, Finegold SM: Epidemiologic aspects of Clostridium difficile-induced diarrhea and colitis. The American Journal of Clinical Nutrition. 1980, 33: 2533-2538.
-
McFarland LV, Stamm We: Review of Clostridium difficile-associated diseases. American Periodical of Infection Command. 1986, 14: 99-109. 10.1016/0196-6553(86)90018-0.
-
Falsey AR, Walsh EE: Manual of Chlamydia pneumoniae. The Periodical of Infectious Diseases. 1993, 168: 493-496.
-
Crosbie Nosotros, Wright Hd: Diphtheria bacilli in floor dust. The Lancet. 1941, I: 656-ten.1016/S0140-6736(00)61019-10.
-
Maule A: Survival of verocytotoxigenic Escherichia coli O157 in soil, water and on surfaces. Symposium Series (Society for Applied Microbiology). 2000, 29: 71S-78S.
-
Abrishami SH, Tall BD, Bruursema TJ, Epstein PS, Shah DB: Bacterial adherence and viability on cutting lath surfaces. Journal of Food Safety. 1994, xiv: 153-172.
-
Kampf Thou, Dietze B, Große-Siestrup C, Wendt C, Martiny H: The microbiocidal action of a new silverish-containing polymer (SPI-Silverish II). Antimicrobial Agents and Chemotherapy. 1998, 42: 2440-2442.
-
Noskin GA, Stosor V, Cooper I, Peterson LR: Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infection Control and Hospital Epidemiology. 1995, 16: 577-581.
-
Wendt C, Wiesenthal B, Dietz E, Rüden H: Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. Journal of Clinical Microbiology. 1998, 36: 3734-3736.
-
Mielke M, Hahn H: Anthropozoonoseerreger ohne Familienzugehörigkeit: Listerien, Brucellen, Francisellen und Erysipelothrix. Medizinische Mikrobiologie und Infektiologie. Edited by: Hahn H, Falke D, Kaufmann SHE and Ullmann U. 2001, Berlin, Springer, 330-340. fourth
-
Gould JC: Pseudomonas pyocyanea infections in hospitals. Infection in hospitals. Edited past: Williams REO and Shooters RA. 1963, Oxford, Blackwell, 119-130.
-
Panagea Due south, Winstanley C, Walshaw MJ, Ledson MJ, Hart CA: Environmental contamination with an epidemic strain of Pseudomonas aeruginosa in a Liverpool cystic fibrosis heart, and written report of its survival on dry surfaces. Journal of Hospital Infection. 2005, 59: 102-107. 10.1016/j.jhin.2004.09.018.
-
Robertson MH: Survival of Southward. typhimurium in flooring dust: a possible reservoir of infection in institutions. Public Health. 1972, 97: 39-45.
-
Nass W: Zur Überlebensdauer von Shigellen auf Plastikwerkstoffen. Zeitschrift für die gesamte Hygiene. 1977, 23: 395-397.
-
Islam MS, Hossain MA, Khan SI, Khan MN, Sack RB, Albert MJ, Huq A, Colwell RR: Survival of Shigella dysenteriae type 1 on fomites. Journal of Wellness, Population, and Nutrition. 2001, nineteen: 177-182.
-
Wagenvoort JH, Sluijsman W, Penders RJ: Meliorate environmental survival of outbreak vs. desultory MRSA isolates. Journal of Hospital Infection. 2000, 45: 231-234. 10.1053/jhin.2000.0757.
-
Barua D: Survival of cholera vibrios in food, h2o and fomites. Public Health Papers. 1970, xl: 29-31.
-
Traore O, Springthorpe VS, Sattar SA: A quantitative study of the survival of ii species of Candida on porous and non-porous environmental surfaces and hands. Journal of Practical Microbiology. 2002, 92: 549-555. x.1046/j.1365-2672.2002.01560.x.
-
Gerth HJ: Viren und virale Erkrankungen. Hygiene und Infektionen im Krankenhaus. Edited past: Thofern E and Botzenhart Thou. 1983, Stuttgart, Fischer, 55-106.
-
Sizun J, Yu MW, Talbot PJ: Survival of human coronaviruses 229E and OC43 in pause and after drying on surfaces: a possible source of hospital-caused infections. Journal of Hospital Infection. 2000, 46: 55-sixty. 10.1053/jhin.2000.0795.
-
Gagneur A, Legrand MC, Picard B, Baron R, Talbot PJ, de Parscau L, Sizun J: Nosocomial infections due to human coronaviruses in the newborn. Archives of Pediatrics. 2002, 9: 61-69. 10.1016/S0929-693X(01)00696-0.
-
Duan SM, Zhao XS, Wen RF, Huang JJ, Pi GH, Zhang SX, Han J, Bi SL, Ruan Fifty, Dong XP: Stability of SARS coronavirus in man specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and Environmental Sciences. 2003, 16: 246-255.
-
Roger Chiliad, Faix MD: Survival of cytomegalievirus on environmental surfaces. The Journal of Pediatrics. 1985, 106: 649-652. x.1016/S0022-3476(85)80096-2.
-
Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynhard JE: Survival of hepatitis B virus afterwards drying and storage for one week. The Lancet. 1981, I: 550-551. 10.1016/S0140-6736(81)92877-iv.
-
Barré-Sinoussi F, Nugeyre MT, Chermann JC: Resistance of AIDS virus at room temperature. The Lancet. 1985, Ii: 721-722. ten.1016/S0140-6736(85)92955-one.
-
von Rheinbaben F, Wolff MH: Handbuch der Virusdesinfektion. 2002, Berlin, Springer
-
Tjotta Eastward, Hungnes O, Grinde B: Survival of HIV-1: activity afterwards disinfection, temperature and pH changes, or drying. Journal of Medical Virology. 1991, 35: 223-227.
-
Nerurkar LS, West F, May Thousand, Madden DL, Sever JL: Survival of canker simplex virus in h2o specimens collected from hot spa facilities and on plastic surfaces. The Journal of the American Medical Association. 1983, 250: 3081-3083. ten.1001/jama.250.22.3081.
-
Brady MT, Evans J, Cuartas J: Survival and disinfection of parainfluenza viruses on environmental surfaces. American Periodical of Infection Command. 1990, eighteen: eighteen-23. 10.1016/0196-6553(90)90206-viii.
-
Pirtle EC, Beran GW: Virus survival in the surroundings. Revue Scientifique et Technique. 1991, 10: 733-748.
-
Roden RBS, Lowy DR, Schiller JT: Papillomavirus is resistant to desiccation. The Journal of Infectious Diseases. 1997, 176: 1076-1079.
-
Schoenbaum MA, Freund JD, Beran GW: Survival of pseudorabies virus in the presence of selected diluents and fomites. Journal of the American Veterinary Medical Association. 1991, 198: 1393-1397.
-
Reed S: An investigation of the possible manual of rhinovirus colds through straight contact. Journal of Hygiene, London. 1975, 75: 249-258.
-
Mahnel H: Experimentelle Ergebnisse über die Stabilität von Pockenviren unter Labor- und Umweltbedingungen. Zentralblatt für Veterinärmedizin. 1987, 34: 449-464.
Pre-publication history
-
The pre-publication history for this paper tin be accessed here:http://www.biomedcentral.com/1471-2334/half-dozen/130/prepub
Acknowledgements
The authors declare that they have no acknowledgements.
Author information
Affiliations
Respective author
Additional data
Competing interests
GK is a paid employee of Bode Chemie GmbH & Co. KG, Hamburg, Germany.
Authors' contributions
All authors contributed to the conception, review of studies, and analysis of data. All authors were involved in drafting and revising the manuscript. All authors approved the last version of the manuscript.
Authors' original submitted files for images
Rights and permissions
This commodity is published under license to BioMed Primal Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/two.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and Permissions
About this commodity
Cite this article
Kramer, A., Schwebke, I. & Kampf, Yard. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6, 130 (2006). https://doi.org/x.1186/1471-2334-vi-130
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1471-2334-6-130
Keywords
- Rota Virus
- Nosocomial Infection
- Hand Hygiene
- Clostridium Difficile
- Serratia Marcescens
harringtoninving84.blogspot.com
Source: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-6-130
0 Response to "How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review"
Post a Comment